





22-13: Visible-light-induced nickel-catalyzed radical cross-couplings to access α-aryl-α-trifluoromethyl alcohols
Feng Chen, Xiu-Hua Xu, Lingling Chu and Feng-Ling Qing*
Org. Lett. 2022, 24, 9332−9336. DOI: 10.1021/acs.orglett.2c03943

22-12: trans-Trifluoromethyltetrafluorosulfanyl Chloride: selective synthesis and reaction with diazo compounds
Xin Zhao, Jia-Yi Shou, Josiah J. Newton and Feng-Ling Qing*
Org. Lett. 2022, 24, 8412-8416. DOI: 10.1021/acs.orglett.2c03540

22-11: Practical synthesis of NFSI derivatives through ArSO2NHF without F2
Yu-Yang Zhang, Xiu-Hua Xu and Feng-Ling Qing*
Chin. J. Chem. 2022, 40, 2956-2962. DOI: 10.1002/cjoc.202200537

22-10: Three-Component Reaction of Pentafluorosulfanyl Chloride, Alkenes and Diazo Compounds and Synthesis of Pentafluorosulfanylfurans
Jia-Yi Shou and Feng-Ling Qing*
Angew. Chem. Int. Ed. 2022, 61, e202208860. DOI: 10.10202/anie.202208860

22-9: A Fruitful Decade of Organicfluorine Chemistry: New Reagents and Reactions
Feng-Ling Qing*, Xin-Yuan Liu*, Jun-An Ma*, Qilong Shen*, Qiuling Song*, and Pingping Tang*
CCS Chem. 2022, 4, 2518-2549. DOI: 10.31635/ccschem.022.202201935

22-8: The radical reaction of ethynylbenziodoxolone (EBX) reagents and pentafluorosulfanyl chloride: new approach to SF5-substituted alkynes
Jia-Yi Shou, Xiu-Hua Xu and Feng-Ling Qing*
J. Fluorine Chem. 2022, 261-262, 110018. DOI: 10.1016/j.jfluchem.2022.110018
22-7: Regioselective oxidative C-H heptafluoroisopropylation of heteroarenes with heptafluoroisopropyl silver
Chao-Lai Tong, Xiu-Hua Xu and Feng-Ling Qing*
Org. Chem. Front. 2022, 9, 4435-4440. DOI: 10.1039/D2QO00787H

22-6: Photoredox catalyzed difluoro(phenylthio)methylation of 2,3-allenoic acids with {difluoro(phenylthio)methyl}triphenylphosphonium triflate
Yao-Yao Zhu, Shuai Liu, Yangen Huang, Xiu-Hua Xu and Feng-Ling Qing*
J. Fluorine Chem. 2022, 257-258, 109969. DOI: 10.1016/j.jfluchem.2022.109969
22-5: Trifluoromethylation with CF3I and other related reagents
Xiu-Hua Xu and Feng-Ling Qing*
The Chemical Transformations of C1 Compounds. Volume 2. Chapter 36
DOI: 10.1002/9783527831883.ch36
22-4: General synthesis of N-trifluoromethyl compounds with N-trifluoromethylamine hydroxyl reagents
Shuai Liu, Yangen Huang, Juan Wang, Xiu-Hua Xu and Feng-Ling Qing*
J. Am. Chem. Soc. 2022, 143, 1962-1970. DOI: 10.1021/jacs.1c12467
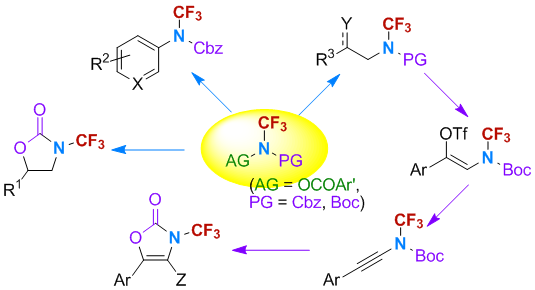
22-3: Photoredox catalyzed C-H trifluoroethylaminationfunction of heteroarenes
Juan Wang, Shuai Liu, Yangen Huang, Xiu-Hua Xu and Feng-Ling Qing*
Chem. Commun. 2022, 58, 1346-1349. DOI: 10.1039/D1CC06688A

22-2: Electrochemical trifluoromethoxylation of (hetero)aromatics with a trifluoromethyl source and oxygen
Yao Ouyang, Xiu-Hua Xu and Feng-Ling Qing*
Angew. Chem. Int. Ed. 2022, 61, e202114048. DOI: 10.10202/anie.202114048

22-1: Iron-catalyzed cyanoalkylation of difluoroenol silyl ethers with cyclobutanone oxime esters
Xiao-Lei Zhu, Yangen Huang, Xiu-Hua Xu and Feng-Ling Qing*
Chin. Chem. Lett. 2022, 33, 817-820. DOI: 10.1016/j.cclett.2021.07.0301


联系方式

友情链接